

Excellence You Can Count On

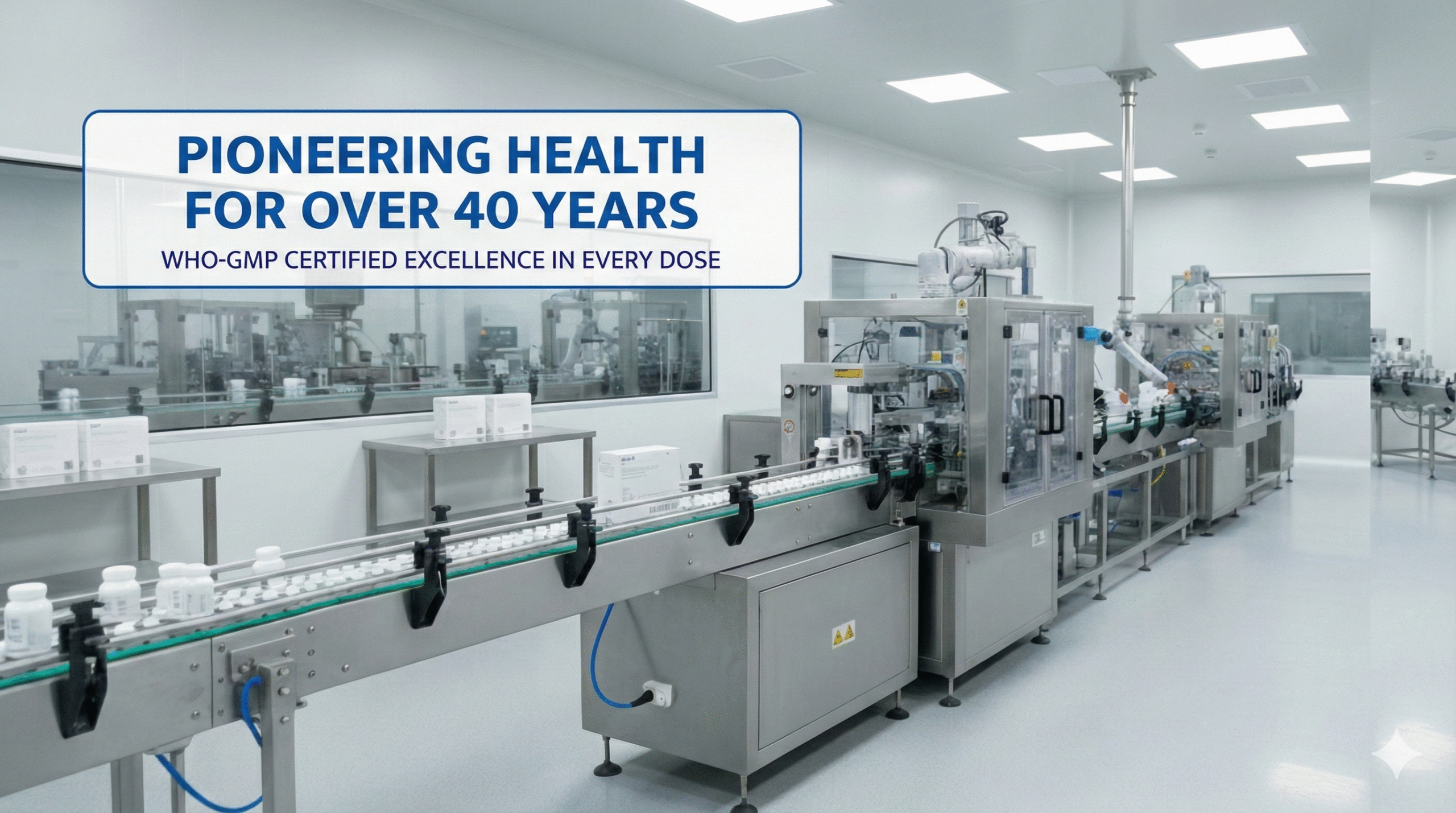
State Of The Art Facility
Cutting-edge WHO-GMP facility: Specialized lines for tablets, capsules, Nano-Shots, injections, and custom forms—with strict controls.
Trusted by Doctors, Approved by Institutions
Endorsed by Leading Physicians and Government Agencies: Reliable, High-Quality Healthcare Delivered.
Quality Control Labs
End-to-End Quality Assurance: Stringent Controls from Raw Inputs to Packaged Products, with No Compromises.
Diversified product portfolio
Our diversified product portfolio spans multiple therapeutic areas, catering to a wide range of healthcare needs. With innovation and quality at the core, we deliver solutions trusted by professionals and patients alike.
Welcome to Unimarck Healthcare Limited
About Us
For over forty years, Unimarck Healthcare Limited has been at the forefront of the
pharmaceutical sector, delivering on a legacy defined by quality, trust, and innovation.

Business Verticals

OTC

Third party manufacturing

Institutional
Where Precision Meets Excellence
Microbiology Laboratory
Our Microbiology Laboratory plays a critical role in monitoring and controlling microbial quality across raw materials, in-process stages, and finished products.
Quality Assurance
Our Quality Assurance system covers every stage of the manufacturing process, from procurement of raw materials to final product release.
Quality control
Our Quality Control system is designed to verify that every product meets predefined specifications before release.
Formulation and Development
(F&D),which form the backbone of our pharmaceutical innovation and product pipeline. Our dedicated F&D team works to design, develop, and optimize formulations that meet both therapeutic needs and regulatory standards.
Manufacturing Capabilities
Our state-of-the-art pharmaceutical manufacturing facilities are engineered to uphold the highest global standards of quality, efficiency, and regulatory compliance. Equipped with cutting-edge infrastructure and advanced technologies, we adeptly address diverse formulation needs across therapeutic categories.
Key Manufacturing Offerings
Technology Transfer & Scale-Up
Seamless progression from formulation development to commercial-scale production.
Validated Processes & Equipment
Rigorous validation protocols guaranteeing reproducibility, reliability, and full compliance at every stage.
Packaging Capabilities
Customized primary and secondary packaging solutions optimized for domestic and international markets.
Skilled Workforce
A highly trained team committed to operational excellence and continuous improvement.
Technology Transfer & Scale-Up
Seamless progression from formulation development to commercial-scale production.
Dedicated Manufacturing Zones
Segregated, contamination-controlled areas for hormonal products, non-β-lactam formulations, injectables, and general tablets/capsules.
Large-Scale Production
High-capacity equipment for cost-effective bulk manufacturing, ensuring unwavering quality consistency.
GMP-Compliant Facilities
Fully designed and operated in accordance with WHO-GMP and stringent international regulatory guidelines.
Awards & Recognitions
Our Clients







Our Brands
Address
Unimarck Healthcare Ltd S-49, First Floor, Janta Market, Rajouri Garden, New Delhi -110027, INDIA.
Call Us
+91 9650194487
Email Us
exports@uhl-pharma.com

